Advisors to the Food and Drug Administration are meeting Thursday to consider giving the go ahead to gene therapy aimed at improving vision for some people with hereditary blindness.
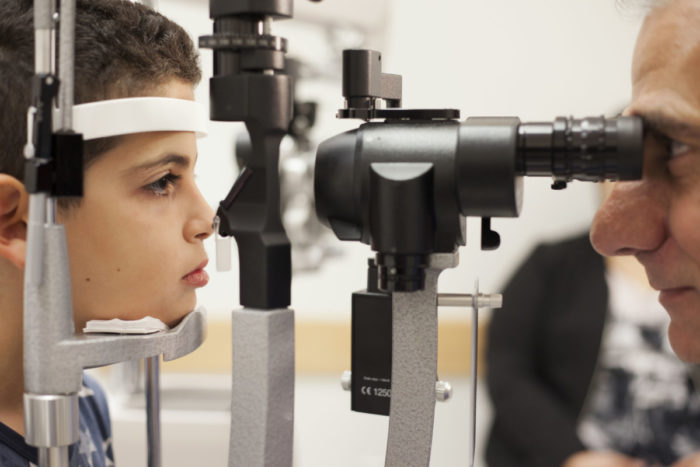
It would be the first gene therapy in the U.S. for an inherited disease and the first in which a corrective gene is given directly to a patient. The eye treatment Luxturna is intended to be given just once and supplies a gene to make a protein needed for sight which is missing in people with a defective gene. A study of 29 patients found it improved vision for nearly all who received it and it seemed safe.
Luxturna is made by Philadelphia based Spark Therapeutics.
Lilian Woo, FOX News.
译文由可可原创,仅供学习交流使用,未经许可请勿转载。












